As the landscape of public health continues to evolve with the ongoing presence of COVID-19, understanding COVID-19 vaccine eligibility has never been more essential. With recent updates in 2025 COVID-19 vaccine guidelines and shifting CDC vaccine recommendations, many are left wondering who qualifies for vaccination. The FDA vaccine approval process has also impacted access, particularly for those under 5, while questions linger over COVID vaccine coverage insurance for various age groups. Pharmacies are on the frontline of vaccine availability, yet not every location is prepared to administer the updated shots. With these factors in play, it is critical for individuals to stay informed and ready to adapt to the changing recommendations surrounding COVID-19 vaccinations.
In light of the ongoing pandemic, clarifying who can receive the COVID-19 vaccine is crucial for ensuring public safety and awareness. As we navigate the complexities of vaccination guidance, it is vital to explore the latest recommendations and requirements set by health authorities. The recent FDA updates and CDC advisories have shaped the conversation surrounding vaccine access across different populations. Understanding the role of insurance in vaccine costs and the participation of local pharmacies in administering vaccines is also increasingly important. With varied eligibility criteria and health recommendations, staying updated is vital for everyone looking to protect themselves and their communities.
Understanding COVID-19 Vaccine Eligibility in 2025
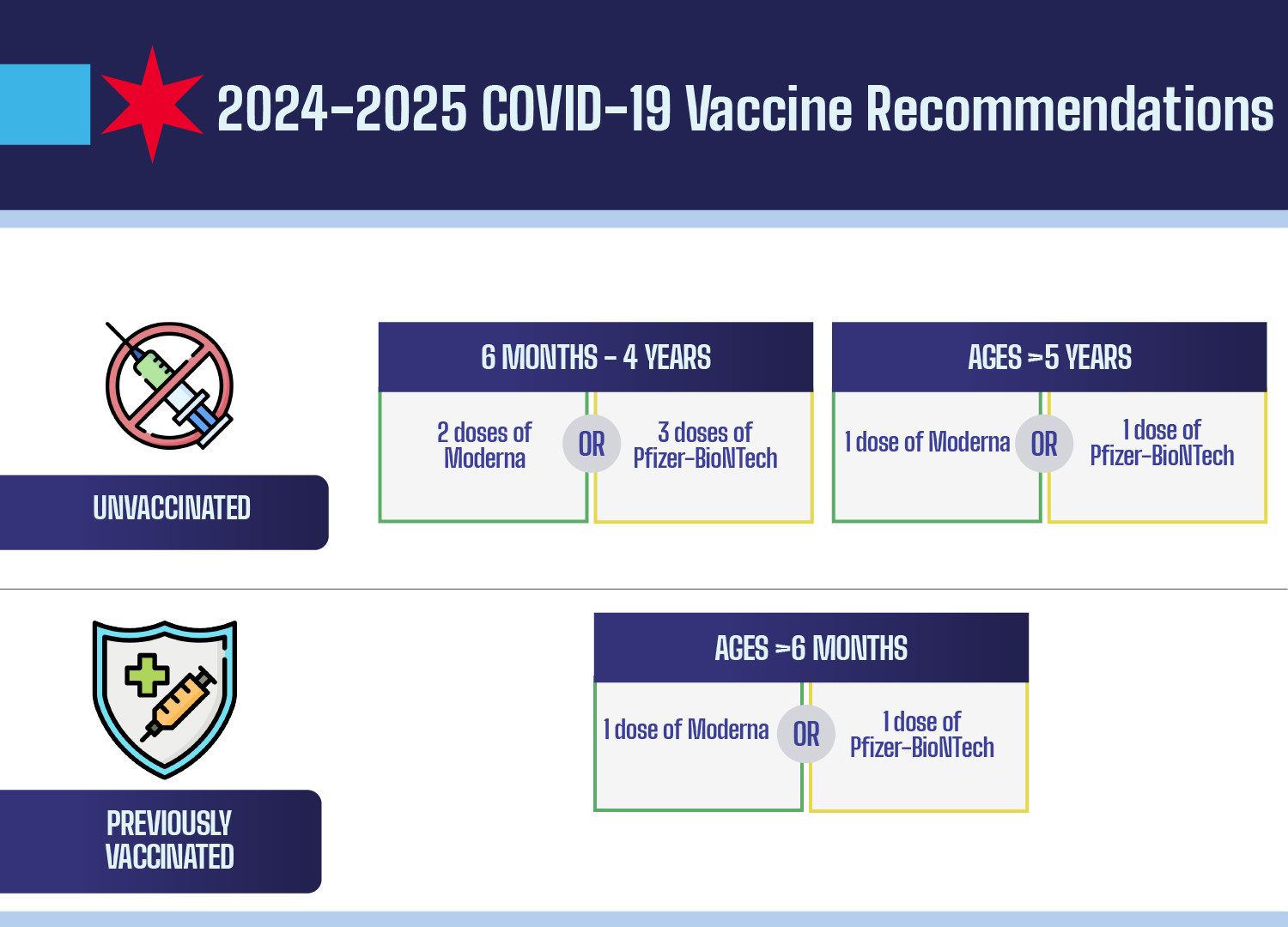
As of 2025, COVID-19 vaccine eligibility has been significantly updated to reflect the changing landscape of the pandemic and emerging data. The FDA has authorized the updated vaccines primarily for adults aged 65 and older, and for younger individuals over 6 months of age who present with certain risk factors such as asthma, obesity, or diabetes. This move marks a shift from previous guidelines that recommended broader vaccination for all individuals older than 6 months. It’s vital for those in high-risk categories to ensure they stay informed about their eligibility and to discuss vaccination options with their healthcare providers.
In contrast to the FDA’s new guidelines, organizations like the American Academy of Pediatrics advocate for broader COVID-19 vaccine eligibility, urging that all children aged 6 months to 23 months receive the vaccine. This discrepancy reflects ongoing debates among health authorities and medical associations regarding the most effective strategies to combat COVID-19. Parents should consult with both their pediatricians and the current CDC recommendations to determine the best course of action for their children regarding vaccination.
Navigating the FDA Vaccine Approval Process for COVID-19
The FDA vaccine approval process is crucial for ensuring the safety and efficacy of COVID-19 vaccines. As federal recommendations evolve, it becomes essential to understand how vaccines are evaluated for public use. The FDA not only considers scientific data on vaccine performance but also monitors ongoing real-world studies. In 2025, the agency specifically tasked its Advisory Committee on Immunization Practices (ACIP) with setting schedules and recommendations, indicating that any changes in vaccine guidelines will likely follow comprehensive review and evidence-based recommendations from trusted healthcare experts.
However, the vaccine approval process has faced scrutiny and controversy, especially with recent changes in leadership and membership within ACIP. Individuals seeking the updated COVID-19 vaccines should be aware that the changing dynamics may influence future vaccine guidelines and availability. It’s recommended to stay informed through official FDA announcements and CDC updates, which can provide clarity on how approvals may shift and the implications for various population segments.
CDC Vaccine Recommendations and their Impact on Healthcare
The CDC vaccine recommendations play a vital role in shaping public health policy and vaccine distribution strategies across the United States. In 2025, these recommendations are undergoing significant scrutiny, particularly following the announcement that they no longer universally recommend COVID-19 vaccination for pregnant women and healthy children. While many medical organizations contend this guidance is too narrow, the impact of CDC recommendations extends beyond just who should receive the vaccine—it also affects insurance coverage and pharmacy accessibility.
Health professionals and parents alike are worried that these updated CDC guidelines might reduce vaccination rates among vulnerable populations. Organizations such as ACOG emphasize the continued vaccination of all pregnant individuals, citing the heightened risk of COVID-19 complications during pregnancy. Understanding and navigating CDC guidance is crucial for individuals and healthcare providers to ensure the effective management of public health in a post-pandemic world.
The Importance of Pharmacy Vaccine Availability
Pharmacy vaccine availability has been a crucial aspect of COVID-19 vaccination efforts throughout the pandemic. As of 2025, nearly 90% of the U.S. population who received the vaccine last year did so at pharmacies, underscoring their essential role in the vaccination strategy. However, with updated CDC recommendations, eligibility to receive vaccines at local pharmacies may become more restrictive, impacting access for many who wish to be vaccinated. In various states, pharmacists are limited to administering vaccines only to specified eligible groups, according to federal guidelines.
Individuals interested in obtaining their COVID-19 vaccines at pharmacies should proactively contact their local drugstore to learn about current policies and restrictions. Those who may not meet the essential eligibility criteria outlined by the FDA could encounter barriers to obtaining the vaccine, prompting a need for increased healthcare provider guidance and community health resources to ensure every individual remains protected against COVID-19.
Insurance Coverage for COVID-19 Vaccines: What to Expect
Understanding insurance coverage for COVID-19 vaccines is key for anyone planning to get vaccinated in 2025. Almost all healthcare payers are mandated to cover vaccines recommended by the CDC without any fees. However, there is uncertainty regarding coverage for those not classified as high-risk or outside of the current federal guidelines. Individuals without insurance may face out-of-pocket expenses for vaccines, which could total around $140, depending on the specific situation.
To prevent financial barriers to vaccination, it’s advisable for individuals to reach out to their insurance providers for detailed information about their coverage plans. As the CDC and FDA guidelines adapt over time, maintaining communication with insurers will help clarify how coverage policies may change and ensure that those seeking vaccination can effectively navigate the evolving healthcare landscape.
Examining the Evolving Guidelines for Children’s Vaccination
With the landscape of COVID-19 vaccinations changing in 2025, families must stay attuned to the evolving guidelines regarding children’s vaccination. The FDA currently recognizes vaccination for young individuals primarily based on their health risks, but many pediatric health organizations, such as the AAP, argue for universal vaccination for all children aged 6 months to 23 months. This divergence in opinions requires parents to assess the health of their children in light of ongoing discussions about vaccinations.
Healthcare providers play a critical role in guiding families through these recommendations, helping them to weigh the benefits of vaccinating against the backdrop of evolving public health guidance. Protecting vulnerable groups, educating parents, and fostering open discussions about vaccination are key components in managing children’s health as it relates to COVID-19.
Long COVID and Vaccination: Addressing Family Concerns
As concerns about Long COVID continue to rise, vaccination emerges as a critical preventive measure for families. Reports indicate that vaccination can significantly reduce the risk of Long COVID in both children and adults, prompting healthcare organizations to advocate for vaccinations even among those who may not exhibit high-risk characteristics. Families are encouraged to remain vigilant regarding the ongoing research surrounding Long COVID and its potential implications.
Doctors such as Sean O’Leary emphasize the necessity of immunization not only to protect against severe COVID-19 but also to combat the long-term effects of the virus that may persist beyond initial recovery. By discussing these risks with healthcare professionals, families can make informed decisions about vaccinations that prioritize both immediate and long-term health outcomes.
The Role of Healthcare Providers in Vaccination Decisions
Healthcare providers serve a crucial role in guiding patients and their families through vaccination decisions, considering the latest recommendations and individual health circumstances. In 2025, amid changing guidelines and public sentiment, many doctors advocate for informed discussions about COVID-19 vaccinations, particularly for individuals categorically defined as low risk. Providers can help bridge the gap in information by addressing vaccine-related concerns and discussing the importance of vaccinations in the context of overall health.
In practical terms, this means that patients should feel empowered to bring their questions to their healthcare visits, especially concerning vaccine eligibility and potential benefits. As COVID-19 vaccinations evolve alongside recommendations from organizations like the CDC and FDA, ongoing dialogues with healthcare professionals will shape personal decisions about vaccination.
Anticipating Future Changes in COVID-19 Vaccination Policies
As we move through 2025, anticipating changes in COVID-19 vaccination policies will be essential for individuals wishing to safeguard their health. Historical trends suggest that policymakers and health authorities will adapt guidelines based on emerging data, public health needs, and community response rates. Keeping abreast of these shifts can prepare families and individuals for expectations surrounding vaccine availability, eligibility, and insurance coverage.
Active participation in health discussions and engagement with local healthcare providers will also provide insights into how upcoming changes may affect personal health decisions. Whether it’s through following CDC updates or consulting with doctors, staying informed will be crucial in a landscape that continues to evolve as we learn more about COVID-19.
Frequently Asked Questions
What are the 2025 COVID-19 vaccine eligibility requirements according to the CDC?
As of now, the CDC recommends the COVID-19 vaccine primarily for individuals aged 65 and older, and for adults and children over 6 months who have risk factors for severe COVID-19. For specific vaccine eligibility guidelines, it’s best to consult the latest CDC recommendations.
Will the FDA approve COVID-19 vaccines for all ages in 2025?
Currently, the FDA has approved updated COVID-19 vaccines only for individuals aged 65 and over, and younger individuals with certain risk factors. Children under 5 can only receive the Moderna vaccine, as the Pfizer vaccine is no longer authorized for that age group.
Are COVID-19 vaccines covered by insurance for all eligible patients in 2025?
Most health care payers are required to cover COVID-19 vaccines at no cost when they are recommended by the CDC and ACIP. However, as guidelines change, it is important to check with your insurance provider regarding coverage specifics.
Where can I receive the COVID-19 vaccine in 2025?
Vaccination for COVID-19 is widely available at many pharmacies across the U.S. However, availability at local pharmacies can vary based on state regulations and whether the pharmacy follows CDC or ACIP recommendations.
Are there differing COVID-19 vaccine guidelines from medical associations?
Yes, several medical associations such as the American Academy of Pediatrics recommend vaccinations for children aged 6 months to 23 months, which contradicts current federal guidelines that are more restrictive.
How does the FDA’s vaccine approval process impact COVID-19 vaccine eligibility in 2025?
The FDA controls the approval of COVID-19 vaccines based on safety and efficacy data, and as such, its decisions significantly affect who is eligible to receive the vaccine.
Will there be ongoing confusion about COVID-19 vaccine eligibility?
Yes, the changing federal recommendations and varying guidelines from medical associations have resulted in confusion regarding COVID-19 vaccine eligibility among providers, patients, and insurers.
What should I know about pharmacy vaccine availability for COVID-19 in 2025?
Pharmacy availability of the COVID-19 vaccine may be limited based on CDC and state regulations. Many pharmacies may not offer the vaccine unless explicitly recommended by federal guidelines.
| Key Points |
|---|
| COVID-19 Vaccine Eligibility Summary: Updated FDA recommendations include authorization for: – Adults 65 and older – Children over 6 months with risk factors (asthma, obesity, diabetes) – Only Moderna approved for kids under 5. Pfizer no longer approved for this group. |
| Conflicting Guidance: – CDC shifted recommendations, now not advising vaccines for pregnant individuals/healthy children. – AAP maintains vaccination for children 6 months – 23 months is crucial. – ACOG recommends vaccination for all pregnant and breastfeeding women due to increased risk of severe illness. |
| Vaccination Recommendations: – Experts suggest moving away from universal vaccination; high-risk groups should still consider vaccination. – High-risk groups include unvaccinated children over 2, pregnant women, and immunocompromised individuals. |
| Insurance Coverage: – Most insurers covering vaccines at no cost if recommended by CDC/ACIP. – Coverage may change based on evolving federal recommendations, individuals should verify with insurers. |
| Vaccine Accessibility: – High likelihood of availability at local pharmacies, which is currently under review depending on adherence to federal recommendations. |
Summary
COVID-19 vaccine eligibility has become increasingly complex in light of recent federal updates. As of now, the FDA has authorized COVID-19 vaccinations for certain populations, primarily older adults and younger individuals at risk for severe illness. However, conflicting guidance from organizations such as the CDC, AAP, and ACOG has created uncertainty regarding who should be vaccinated. It is crucial for individuals to consult medical professionals and verify insurance coverage before seeking vaccination, particularly in the shifting landscape of public health recommendations.